
In the first part of this two part series we discussed how the sheer amount of energy coupled by its cheap cost enabled society to increase the amount of work that could be produced at a much reduced cost. It was this cheap energy subsidy that made the process of arbitrage between human labour and fossil powered capital very profitable as the price difference between hiring labour ?energy? and capital ?energy? was so vast.
This huge energy subsidy not only provided great wealth to the ruling classes who owned the factories and other capital infrastructure it also enabled the workers to become more materially wealthy as the cost of producing items was reduced drastically. This happened because all work requires energy to transform a basic resource into a commodity that is of economic value. If the energy cost is reduced and the supply of energy is greatly increased then the amount of work that can be achieved by a society will greatly expand and thus the items produced well sell at lower price due to the principles of supply of demand. What is important when discussing these matters of energy availability and will become increasingly important going forward is the concept of net energy. To understand this concept it would be wise to understand the laws of thermodynamics, more precisely the first law of thermodynamics. This law was originally described as:
?In all cases in which work is produced by the agency of heat, a quantity of heat is consumed which is proportional to the work done; and conversely, by the expenditure of an equal quantity of work an equal quantity of heat is produced.? ? Rudolf Clausius, 1850[1]
| For the mathematically inclined the following formula captures the law of conservation as any heat input will equal the work done. ? dU = dQ ? dW[2] where U =Internal energy of system, Q = Heat input, ?W = work done |
To many this statement may seem a little unwieldy and rather abstract but it basically describes the concept that energy cannot be created or destroyed and all energy transactions are merely conversions of one form of energy source to another. This idea is an important one to grasp since we can never actually generate energy; we merely extract it from existing sources.
This statement may seem rather obvious but since terms such as energy production and energy generation are so widespread it is easy to forget this fact. If we were to take such statements as energy production on a literal level they would be a clear violation of the first law of thermodynamics. Perhaps it can be argued this is just an argument over semantics and most people will know you cannot actually create energy but let us not underestimate the power of language and how it can shape conversations and narratives; over time people will believe such statements at face value as true even if they are patently false. This is especially relevant today when we hear so much talk about the US becoming not only energy independence but also becoming the top energy producer in the world.
But I digress, the main thing to take home is no form of energy extraction, be it from coal, nuclear fission (fusion for the optimists) and wind or solar actually generates energy. It just utilises existing energy sources. In the above examples energy is extracted either through nuclear fission/fusion reactions, potential chemical energy in coal/oil/gas or wind and solar energy. Another concept that will come from this basic idea and one that perhaps even more relevant is that it takes energy to get energy. This concept while important is something that rarely (if ever) gets discussed in the mainstream media. Much of the talk about oil, coal or gas ?production? only refers to the amount of total energy that can be obtained from burning or utilising a given resource. This amount is merely the gross energy obtained from the ground. If we wish to determine the amount of useful energy available for greater society however we need to subtract the amount of energy used to obtain the resource in the first place. This is because it is only this energy that gets to be used by society for other economy activities. Thus for us to work out net energy we subtract the gross energy by the energy needed to extract the resource as described in the formula.
Gross energy = Total amount of energy obtained from energy source.*
Net energy = Gross energy ? Energy required to obtain energy source.
* = Energy maybe expressed in other ways such as barrels of oil or million short tons of coal.
As a note net energy should not be confused with the similar but different term EROEI (Energy Return On Energy Invested) which describes the potential energy return from an energy source. The terms maybe used interchangeably by other commentators in the blogosphere but it would be a mistake to think they are the same thing. The way to calculate EROEI is quite different as demonstrated below:
EROEI =Gross energy / Energy required to obtain energy source.
or
EROEI = (Net energy/ Energy required to obtain energy source.) + 1
NOTE: Net energy is the energy available to society and thus something greater society would be interested in whereas EROEI is more of a potential concern for the person who wishes to see a return on their investment.
Since it is only net energy that gets used by the greater economy it is this value that we should be interested in knowing about rather than the gross amount. For example it is quite possible for our total gross energy to increase while our total net energy is actually in decline. If this were to happen we could easily see a scenario where we are getting materially poorer even though our total energy output is increasing. This process of increasing gross energy but declining net energy comes about due to the principle of low hanging fruit. That is the easiest and most favourable economic sources of energy ? which yield the highest net energy ? are extracted first and as those resources are depleted we move onto progressively worse and worse sources. It is this fact that the decline in net energy will be much steeper than gross energy decline.
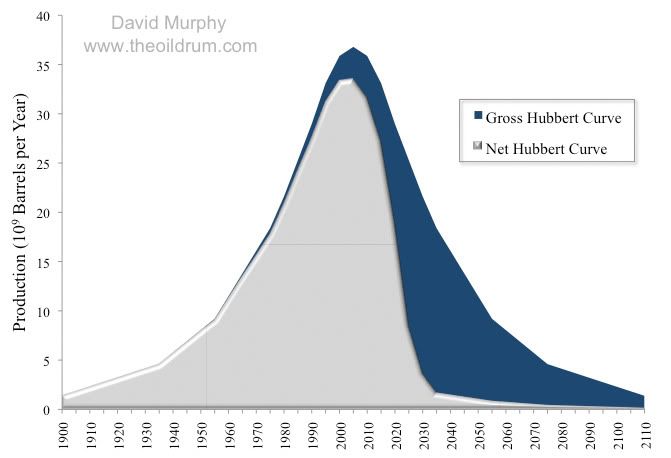
This trend of declining net energy has likely already past as the newest sources of energy, a lot of which is touted as the energy source that will give the US energy independence actually yield poor amounts of new net energy. This is despite the fact this new energy sources (shale oil/gas) increase the amount of gross energy expended by the economy quite substantially. As a result from an economic standpoint these new sources of energy will deliver less economic benefit to society than would otherwise be believed as the extra net energy available will be more limited. It this reason why we should exercise caution when listening to claims that these sources of energy can offer a panacea to our economic troubles. In fact when hearing such claims it is useful to know the energy returns on energy for various sources as while this is not net energy which is ultimately the most important metric to gauge the EROEI can still provide an insight on how useful these sources will be:

As the graph clearly demonstrates; early sources of energy yielded a very high return of energy on energy invested. These high returns came about because early sources of energy such as shallow coal mines did not need much capital investment to extract the resource furthermore once these resources were obtained they would yield high quality energy such as light sweet oil or in the case of coal high quality anthracite. It is this reason in fact why net energy or EROEI has largely been ignored as historically gross energy for all intents and purposes equalled net energy.
In recent years however this has not been the case and the difference between gross and net energy is sufficiently large to warrant greater attention. Moreover another troubling fact to take note is that once EROEI reaches about 10:1 or lower the graph goes into steep decline. This steep decline means the available net energy that can be used by society will begin dropping at an alarming rate if current trends of extracting lower quality energy sources continue. If we take a recent major discovery of shale oil we discover the EROEI for this resource is 5.[3] In net energy terms this represents 20% of the total gross energy being used to extract the energy source. So from this information we can say that if all existing resources of energy were replaced by sources that were to yield returns equivalent to shale oil then our total net energy available to society would decline by 20%. This assumes there are no efficiency gains in how we utilised this energy and total gross energy remained constant. If that is the case then this would effectively mean we are 20% poorer as less energy would be available for economic transactions.
Off course what is more likely to happen is the EROEI and thus net energy will decline even faster than suggested in the previous paragraph as worse and worse sources of energy come online to replace existing high EROEI resources. As a result we are likely to see a steep decline in net energy available to society and we can say with some certainty that this decline in net energy will be faster than the rate of efficiency gains which has been around 1.7-3.0% per annum since the 1970s.[4] [5]
What is more due to the rebound effect (see this article for more info) it is likely that any efficiency gains made will need to exceed declines in available net energy by a few percentage points each year if we want sustain economic growth (which is a requirement for the financial system to remain stable). This all seems unlikely particularly if we consider that energy efficiency and conservation strategies will see diminishing rates of return as it becomes harder to increase energy efficiency after each progressing year. After all no economic activity can ever achieve 100% efficiency. Speaking of 100% efficiency this leads on nicely to the second law of thermodynamics which states:
?That the entropy of an isolated system never decreases, because isolated systems spontaneously evolve towards thermodynamic equilibrium ? the state of maximum entropy.?
Like the first law, this sentence describing the second law can seem a little unwieldy. In fact it is best to breakup this statement into two sentences as the quotation above addresses two very relevant points the first of which is displayed below:
?No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion into work.? ? William Thomson, 1st Baron Kelvin, 1851[6]
In other words no energy transaction can ever be 100% efficient meaning some energy will always be lost when converting energy into some form of work. This point while blatantly obvious is often overlooked in the contexts of economics and broader society. It is this reason why there will always be a limit to amount of economic growth that can be realised as the planet has a finite number of resources and there are limits to the amount of efficiency that can be achieved. Another major consequence that does derive from the second law of thermodynamics comes from this statement:
?Heat can never pass from a colder to a warmer body without some other change, connected therewith, occurring at the same time.? ? Rudolf Clausius, 1850[7]
While at first glance, the concept of heat transfers may seem a little out there this statement does pertain to one important idea. That is over time all bodies and structures go from a process of order to disorder. For those that are really curious about this process and wish to learn more about the exact mechanics of this process please look up entropy. If this idea still seems a bit out there consider the fact that all structures, be it capital or labour, degrade over time and require maintenance to allow proper functioning. This maintenance always requires energy and thus some resources will always be needed to maintain current capital or labour.
?
?
| The entropy of a system can be calculated by applying the following formula: dS = dQ / T[6] where S = entropy, Q = Heat input, T = Temperature of system. If we use the formula from the first law of thermodynamics and rearrange the formula shown above this statement can be derived: dU + dW = TdS ?*4 basic thermodynamic relationships develop out of this math ? Internal Energy dU=TdS-PdV+??dN Enthalpy (Heat) dH=TdS+Vdp+??dN Hemholtz Free Energy dF=-TdS-pdV+??dN Gibbs Free Energy dG=-TdS+Vdp+??dN ? If you do a little basic algebra, you get dG=dH-TdS This equation gives the Free Energy available in a system as a function of the Heat Content, the Temperature and the Entropy in the system. Only reactions with positive dG can go forward without Heat Input. *from RE |
In the case of labour humans need food to stay alive and remain functioning while capital requires some energy inputs to prevent it from degrading and evening breaking down over time. What is more the greater the complexity of a structure the more energy will be required for maintenance. This is because a more complex structure has a greater degree of order and since things naturally go from a state of order to disorder then more energy will be needed to prevent overcome this natural process.
This is another weakness with applying the logic that technology will save the day as increasing the complexity of technology not only increases the existing maintenance cost due to the second laws of thermodynamics; the cost of producing such items increases as more energy per unit weight are needed in the manufacture of the product. To demonstrate this example a car requires something in the region of 12-25 barrels of oil to build a car depending on the weight of the car but a computer ? on a weight by weight basis ? requires 10 times the amount of energy to manufacture.[9] A similar cost will be borne in maintaining these two pieces of capital. While in theory there can be energy savings on a production basis as less energy will be consumed despite the increased weight for weight costs (we do not need 1000kg+ worth of computers after all) what will increase significantly are maintenance costs of more complex infrastructure. To give a better idea of this concept at work consider healthcare. As the capital becomes increasingly complex capital then the maintenance costs will rise for the reasons described above.
To summarise this article and the one before it; when we wish to engage in a discussion on energy we need to be aware of range of things. First we need to understand the sheer amount of energy fossil fuels provide. It is truly immense and is a miracle resource and there needs to be a greater appreciation just how much energy they can deliver. From this we can truly grasp the scale of the task an energy transition (if it is even possible) will be. It is seems unlikely to me any combination of renewable or nuclear energy can fill the gap left by fossil fuels. That is not to say renewables cannot make life easier, they do have their uses but we would be setting our expectations too high if we expect them to maintain our current industrial lifestyles.
The other important points that need to be considered is the point we should be interested in not only the quantity of energy delivered but also the quality of energy. At the end of the day it is net energy or EROEI we are really interested in as it is this energy that gets used for greater society. Finally we need to be aware that due to the increasingly complexity of society our maintenance costs will rise due to the second law of thermodynamics so these costs need to be accounted for.
Doomstead Diner
Source: http://peakoil.com/generalideas/energy-part-ii-eroei/
rosie o donnell soda bread recipe vanderbilt evan mathis staff sgt. robert bales jason russell norfolk state
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.